Understanding ANSI/AAMI ST108:2023 Water Standards
- Home
- Understanding ANSI/AAMI ST108:2023 Water Standards
Are you in compliance?
In 2023, the Association for the Advancement of Medical Instrumentation (AAMI) has published, ANSI/AAMI ST108:2023 – Water for the Processing of Medical Devices. This new standard replaces AAMI TIR34:2014, the standard that previously set requirements for processing and cleaning of medical devices. This new standard is directed at providing medical professionals with information and requirements specifically for the water used in these processes. The unique usage of this water requires that they meet strict performance standards not only for chemical makeup, but also for microbiological contamination.
From the AAMI website:
Adverse Effects of Poor Water Quality
Water impurities can have adverse effects on medical device processing, including:
- Adverse effects to the PRODUCT:
- Corrosion, pitting, scaling
- Biomass build-up
- Increase microbial load or endotoxin content
- Adverse effects to the PROCESS:
- Decreased effectiveness of detergents
- Degradation of the water system (biofouling or scaling)
- Adverse effect to the PATIENT:
- Infection
- Toxicity
Understanding the Critical Role of Water Quality
Water quality is a pivotal component in the effective cleaning and processing of medical devices. Proper water quality ensures that cleaning agents function at their best and that devices are thoroughly rinsed, minimizing residual contamination. Without water of the specified quality, even the most well-validated procedures can fall short, leading to compromised device safety and performance.
Sterile processing personnel must be vigilant in recognizing water quality issues that could lead to adverse patient outcomes. Awareness of indicators such as unusual residue or inadequate rinsing can be vital in identifying potential water quality problems early on.
Conclusion
The impact of water quality on medical device processing cannot be overstated. Ensuring the use of water that meets specific quality standards is essential not only for the integrity of the devices themselves but for safeguarding patient health. By maintaining high water quality standards, healthcare facilities can enhance the overall efficacy of their sterilization processes.
Water impurities can have adverse effects to medical device processing, including:
- Adverse effects to the PRODUCT:
- Corrosion, pitting, scaling
- Biomass build-up
- Increase microbial load or endotoxin content
- Adverse effects to the PROCESS:
- Decreased effectiveness of detergents
- Degradation of the water system (biofouling or scaling)
- Adverse effect to the PATIENT:
- Infection
- Toxicity
It is important that sterile processing personnel understand the water quality issues that can contribute to adverse patient events and be aware of some of the gross indicators that suggest that there may be problems with the water quality.
The following table details the basic parameters that need to be monitored for the different types of water used in processing.
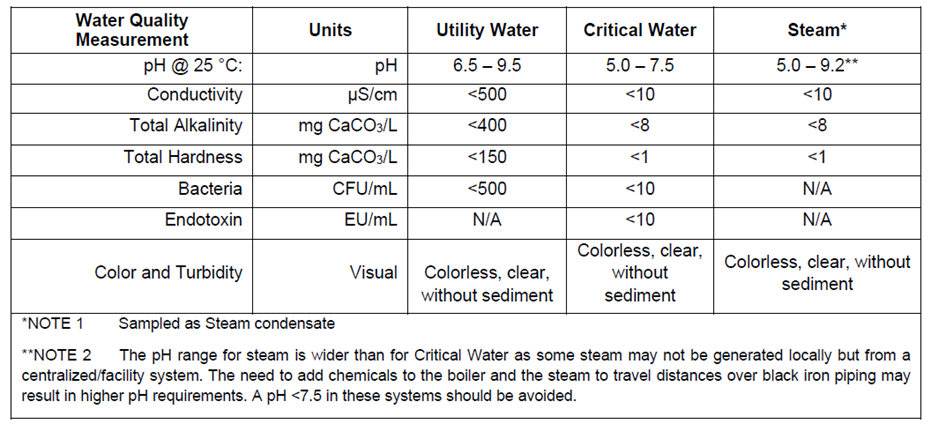
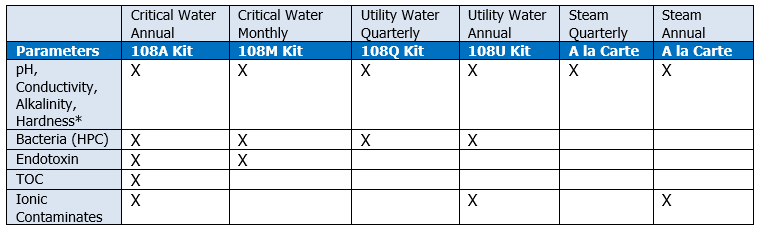
In addition to the general requirements that are monitored on a monthly or quarterly basis depending on type of water (utility, critical or steam) the following ionic contaminates need to be evaluated annually.
American Analytical recommends that a facility should test their water systems according to the standards above that are applicable to water type and usage. We provide full-service laboratory analysis for all the analytes required by ANSI/AAMI ST108:2023.
Endotoxin Testing
Occasionally, endotoxin may be desired or requested outside of regulatory needs, traditionally as a part of the systems validation. American Analytical recommends that a follow up sample should be done shortly after remedial action is taken, typically within 24 hours. Since these numbers represent a point-in-time of testing, it would be prudent to establishing testing on a routine basis to identify trends and potentially prevent an endotoxin issue that may be evolving.
Learn More About Endotoxin Testing With American Analytical Here


